NEWS&EVENTS
Home > News&Events > Company news > Comparison of Oxidation Roasting and Reduction Smelting in Antimony Smelting Rotary Furnace
As a key equipment for antimony smelting, the rotary furnace has essential differences in chemical purpose, temperature, and atmosphere control between the oxidation roasting and reduction smelting stages, which together form a complete metallurgical chain from ore to metallic antimony.
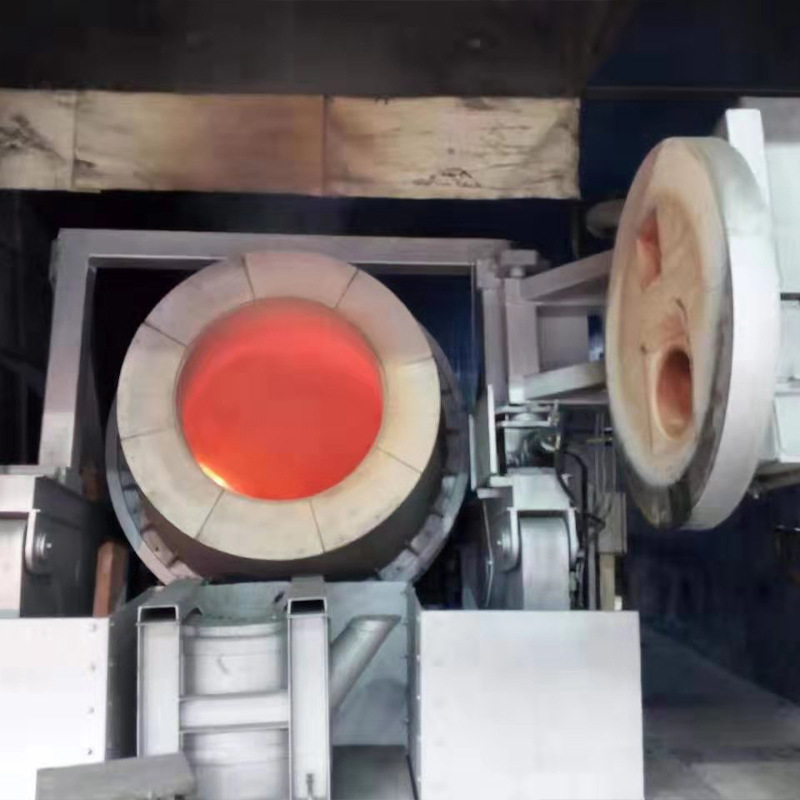
In terms of the main purpose of chemical reactions, the two stages are completely opposite. Oxidative roasting is aimed at antimony sulfide ores (such as stibnite, Sb ₂ S3), with the purpose of converting them into antimony oxide (Sb ₂ O3) that is easy to process later through oxidation desulfurization, and may partially generate antimonates, while separating volatile impurities such as arsenic. And reduction smelting is aimed at the oxidation roasting product (Sb ₂ O3), with the purpose of reducing antimony oxide to metallic antimony under the action of reducing agents, achieving the transformation from compound to elemental metal. In short, the former is "oxidation slag removal", while the latter is "reduction purification to obtain metals".
There is a significant difference between the two in terms of temperature control requirements. The oxidation roasting temperature is usually controlled between 850 ℃ and 1000 ℃. This temperature range requires precise balance: if the temperature is too low, the reaction will not be complete and desulfurization will not be thorough; If the temperature is too high, it may cause severe volatilization loss of antimony oxide (Sb ₂ O3), reduce the recovery rate, and may cause material melting and affect the furnace condition. Reduction melting requires a higher temperature, generally controlled between 1200 ℃ and 1300 ℃. High temperature aims to ensure sufficient reduction reaction and facilitate the good melting of the generated metallic antimony with slag, making it easier to separate and produce crude antimony.
The most crucial difference lies in the control of the atmosphere inside the furnace. Oxidative roasting must be carried out in a strongly oxidizing atmosphere, and excess air or oxygen enriched air must be introduced to ensure that sulfur is fully oxidized into sulfur dioxide (SO ₂) and discharged, and the target oxide is generated. Insufficient atmosphere can lead to the production of substandard intermediate products. On the contrary, reduction smelting requires operation under a strong reducing atmosphere, usually by adding solid carbonaceous reducing agents such as anthracite and coke, and strictly restricting the entry of air to create a carbon monoxide (CO) environment to reduce antimony from oxides. Any mixture of oxidizing atmosphere will cause the metal to re oxidize, severely reducing the yield.
In summary, from oxidation roasting to reduction melting, the operation of the rotary furnace has undergone a fundamental transition from oxidation to reduction, from medium temperature to high temperature, and from oxidizing atmosphere to reducing atmosphere. This precise reverse regulation is the core technical logic of the pyrometallurgical antimony refining process, reflecting a profound understanding and industrial application of the chemical properties of antimony compounds.